Healthcare Professional Confirmation
The information on the page you are about to enter is intended for Healthcare Professionals only.
Return to the Patient Website

A Minimally Invasive Treatment For BPH
The UroLift™ System
Benign prostatic hyperplasia affects over 660 million men worldwide.1,2 While men can select from a variety of treatment options - from medication to major surgery - there has been a gap in the treatment continuum for a less invasive option.
Prostatic Urethral Lift using the UroLift™ System was designed to meet this need. With its unique design and mechanism of action, the UroLift System is a treatment that provides immediate, visible results.3 The procedure can be done in an outpatient or day surgery setting and under general or local anesthesia.4 Patients typically can return home the same day without a catheter4, and experience rapid symptom relief and recovery with low complication rates.3,4 The UroLift System treatment revitalizes the quality of life for patients and improves the BPH therapy experience for physicians.5

The UroLift System Treatment
The UroLift System uses a proven approach to treating BPH that lifts and holds the enlarged prostate tissue out of the way so it no longer blocks the urethra. It is the only leading BPH treatment that does not require cutting, heating, removal, or destruction of the prostate tissue5-9
Treatment with the UroLift System typically takes less than one hour and does not preclude future UroLift System treatments, TURP, or laser procedures.5
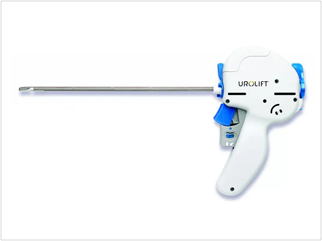
The UroLift Delivery Device & Implant
The UroLift System is comprised of two main components:
- UroLift Delivery Device: inserted transurethrally through a rigid sheath under cystoscopic visualization to reach the targeted area of obstruction. Each delivery device contains one UroLift Implant.
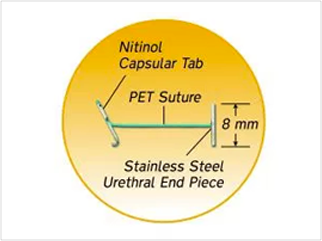
- UroLift Implant: the obstructing prostatic lobes are held apart by small permanent UroLift Implants that are deployed by the delivery device. Each implant is made with common implantable materials: nitinol, stainless steel, and PET suture.
Non-clinical testing has demonstrated that the UroLift Implant is MR Conditional.10 A patient with this device can be safely scanned in an MR system immediately after placement meeting the following conditions10:
- Static magnetic field of 3.0 Tesla or less
- Maximum spatial field gradient of 1,500 Gauss/cm (15 T/m)(extrapolated)
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4 W/kg for 15 minutes of continuous scanning (i.e., per pulse sequence) (First Level Controlled Operating Mode).
Under the scan conditions defined above, the UroLift Implant is expected to produce a maximum temperature rise of 2.4°C after 15 minutes of continuous scanning (i.e., per pulse sequence).
The safety of the delivery system has not been evaluated in the MR environment, and therefore, the delivery system should not be used within the MR environment.
References
1. Berry, J Urol 1984
2. US Census Bureau international database worldwide population estimates for 2020
3. Roehrborn, J Urol 2013
4. AUA Guidelines 2003, 2020
5. Roehrborn, Can J Urol 2017
6. Mirakhur, Can Assoc Rad J 2017
7. McVary, J Urol 2016
8. Gilling, Can J Urol 2020
9. Kadner, World J Urol
10. UroLift System -Instructions for use, data on file